Research That Represents: DiME, Lightship, & Acclinate Collaborate to Support Inclusivity

Only 20% of clinical trial participants are people of color. Less than 45% of participants are women.1 At the same time, the FDA commissioner recently described “meaningful representation” in clinical research as “fundamental to public health.”
The divide between current clinical research practices and those that effectively promote diversity is growing, due in large part to our increased awareness of what works and what doesn’t — what encourages broad participation and what discourages minorities from engaging with the process. That said, there is a critical difference between awareness and action. While many organizations may think it is important to create more inclusive clinical trials, they often lack the resources and the know-how to insert meaningful measures of equity and diversity into their trial design and execution.
In response, the Digital Medicine Society (DiME) has teamed up with industry leaders like Lightship and Acclinate to offer insight, support, and practical resources through the Diversity, Equity, & Inclusion Initiative. These resources are designed to provide actionable steps for transforming your approach to clinical research, and they are the beginning of creating trials that are person-centered and fitted for the future.
Why Take Action?
Now is the ideal time to take action against the lack of diversity in clinical research. Why? First, the representation gap is only growing the more we learn about the vulnerabilities of certain populations, making it increasingly paramount to align our research practices with our cultural realities. For example, Black Americans account for over 35% of kidney failure in the United States, while the number of Latino with kidney failure has increased by 70% in the past two decades.2 Unfortunately, these numbers are not represented in the populations actually participating in research, with less than 10% of both Black Americans and Hispanic Americans enrolled in clinical trials.1
These realities are rooted in mistrust, which is itself the product of years of unethical research and unfair treatment against people of color. Even today, many racial and ethnic minority groups (REMGs) don’t have access to quality healthcare, let alone the time and information to participate in clinical trials. This inequality is further reinforced by the troublesome logistics that often plague underserved neighborhoods and communities, such as a lack of public transportation or overall health literacy.
Additionally, modern technology is making it more feasible than ever to address these gaps in representation. Virtual participation in healthcare is at an all-time high with nearly 40% of U.S. adults taking part in telemedicine, and digitization is creating new options in nearly every corner of the clinical research process3:
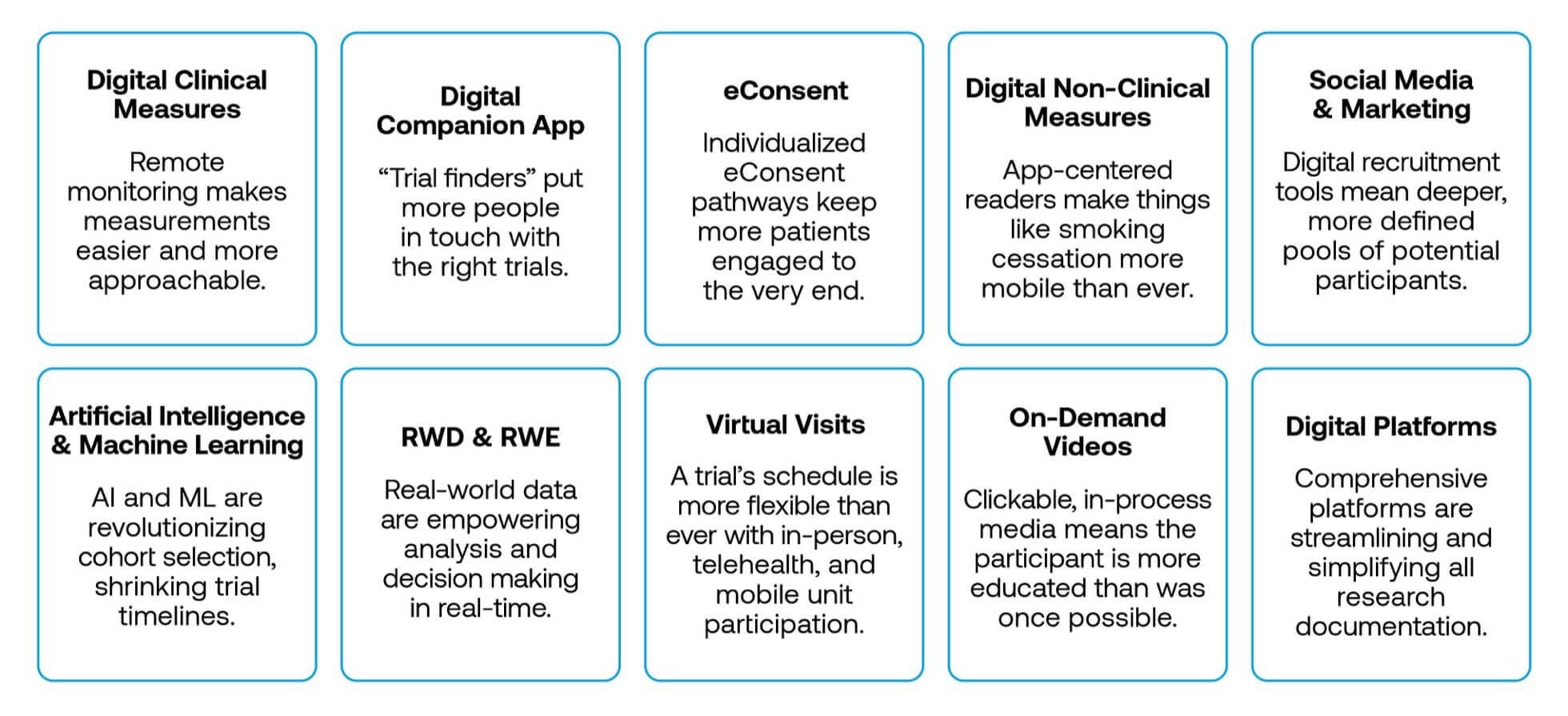
Clearly, technology is opening up many new doors in clinical research. That said, it is important that some of these new doors lead to greater diversity, and this confluence of urgency and ability is exactly what inspired Lightship’s collaboration with DiME.
Sharing Resources. Sharing a Mission.
The Diversity, Equity, & Inclusion Initiative offers the insight and digital tools necessary for developing person-centered strategies that increase representation in clinical trials. DiME encourages organizations to approach every clinical trial and patient community as part of a three-step process:
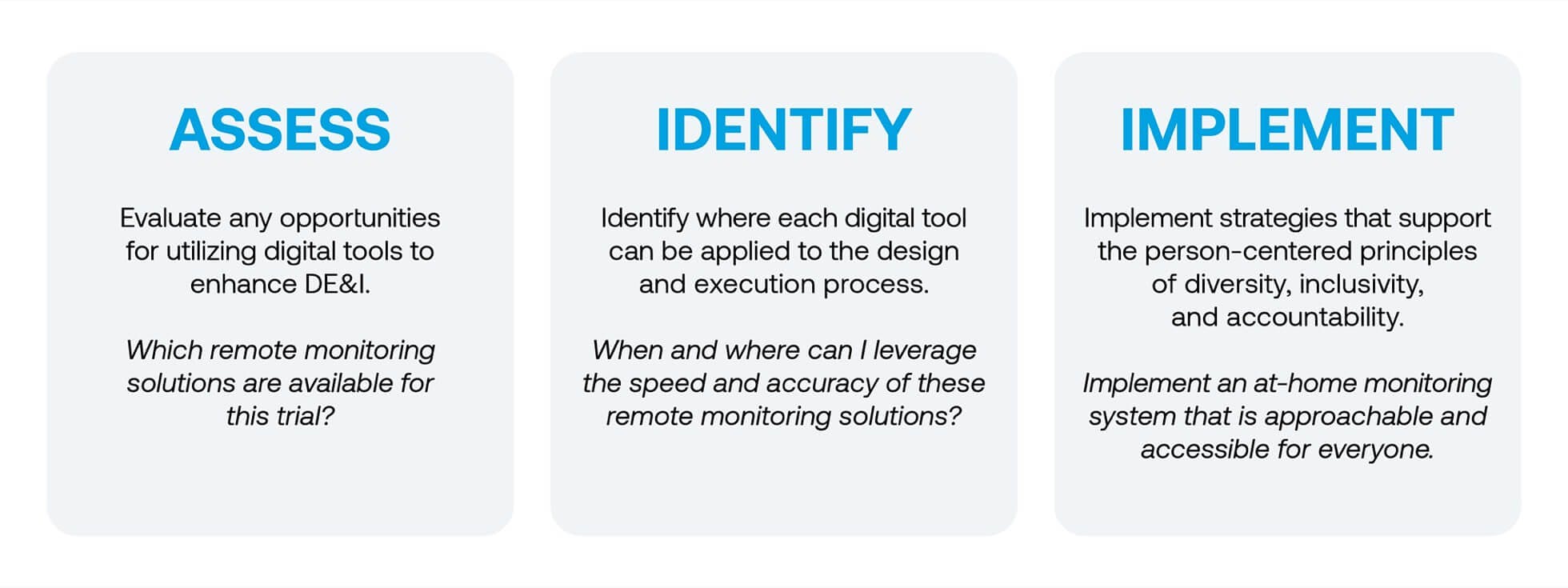
Lightship and Acclinate worked incollaboration with DiME and other industry leaders to develop a package of resources that promotes best practices across the industry, such as the tracking of diversity-related data via a “Diversity Dashboard” and building trust among marginalized communities through local partnerships. Each resource is informed by DiME’s three-step process, fully vetted and enhanced by Lightship and other experts:
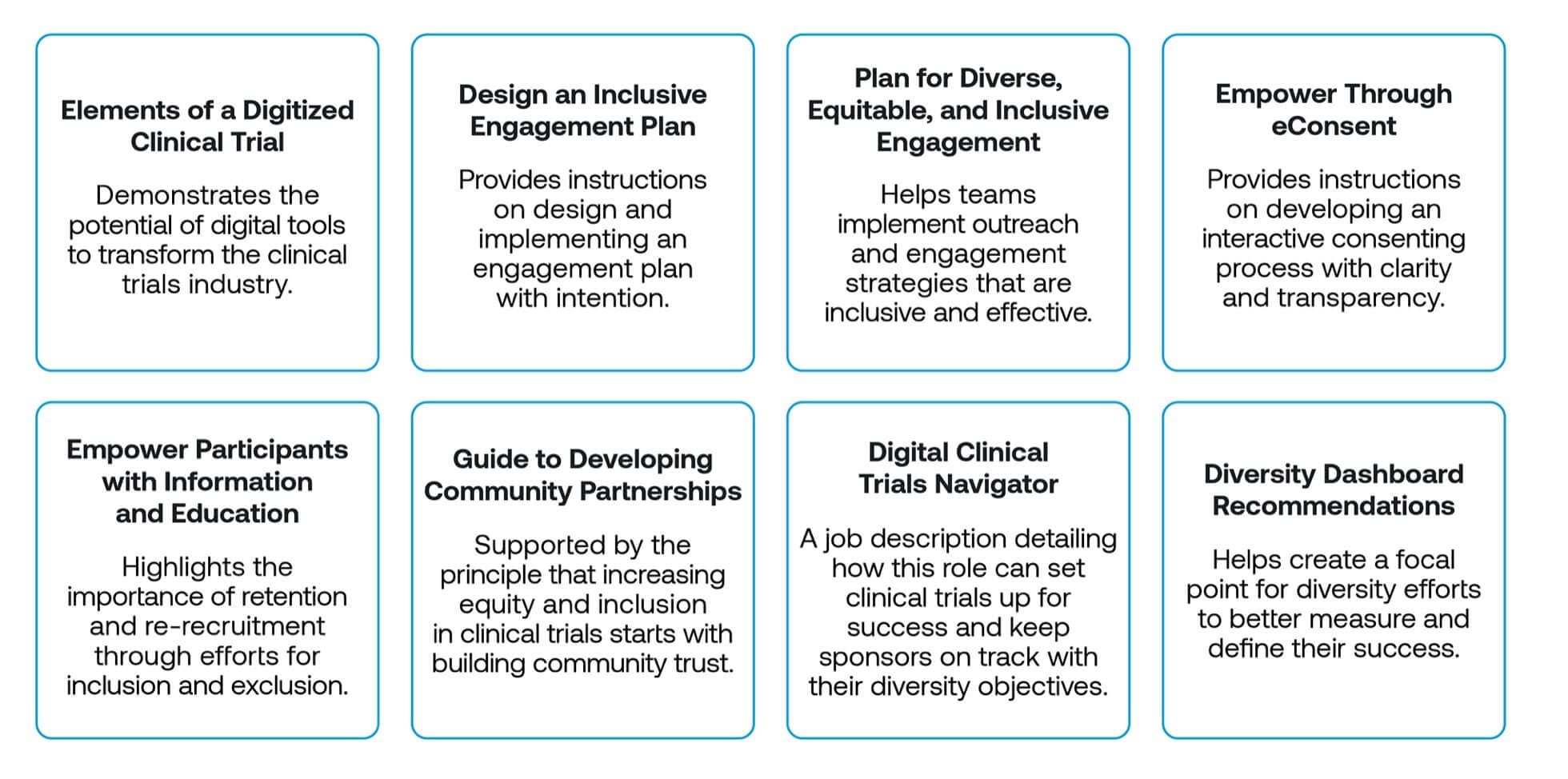
These resources allow you to approach your clinical trials with confidence, knowing that the priorities and tactics behind your overall strategy covers all four levels of engagement: notify, solicit, integrate, and support. You will have provided knowledge and information (NOTIFY), empowered your participants (SOLICIT), developed trust (INTEGRATE), and advanced health equity (SUPPORT), ultimately creating more person-centered clinical trials. But, what do those trials actually look like?
What Defines Person-Centered Trials?
Lightship, along with its collaborative partners at Acclinate (see sidebar), has been dedicated to a people-focused approach to clinical research from the very beginning, so it should come as no surprise that our idea of “person-centered trials” is fully realized and established. Person-centered trials are the only types of trials capable of addressing present disparities, and these trials are defined by three basic tenets: access, choice, and equity.
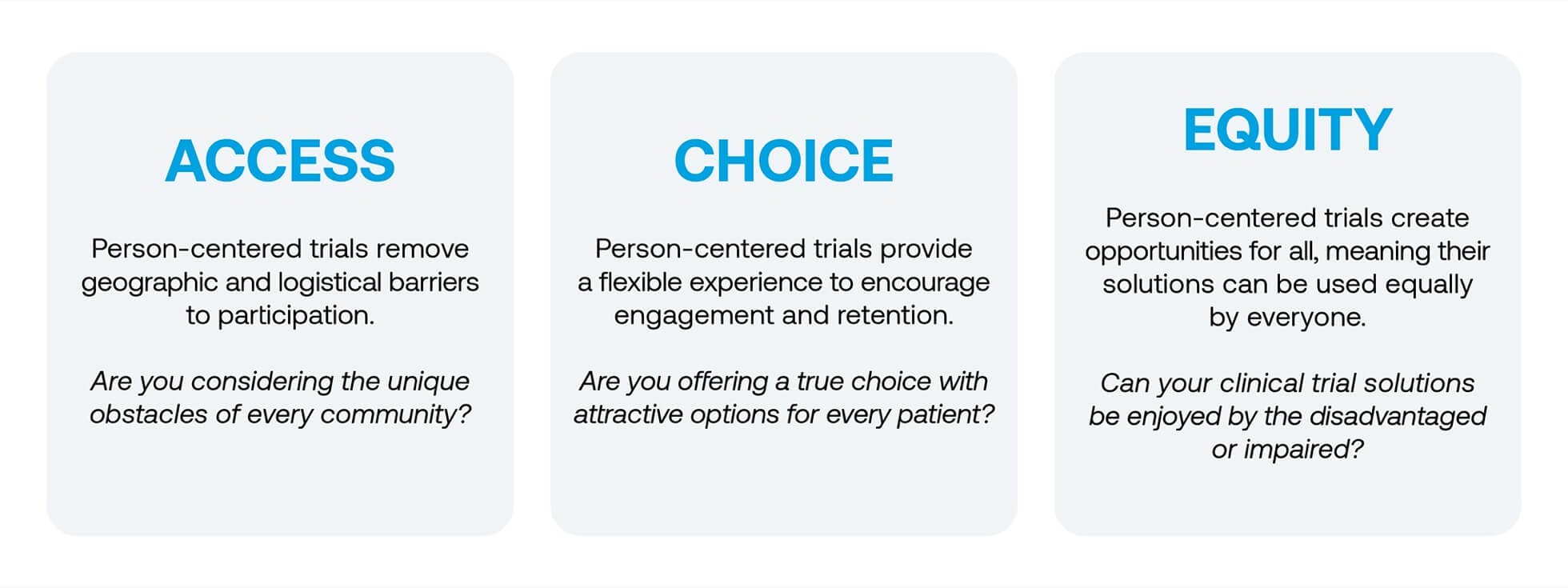
Unfortunately, these areas of consideration, along with the needs of many REMGs, are often neglected in the face of a pressing timeline or budget, which is likely why 86% of clinical trials do not meet their recruitment targets. Of course, digitization is an excellent avenue for addressing these disparities, but it is only the beginning. Highly specialized expertise, diverse leadership, and a deep, practical understanding of different socioeconomic groups are vital to any successful implementation. Achieving this level of understanding is no small feat, and it requires those executing clinical trials to ask some very difficult questions:
- Does my training emphasize cultural sensitivity?
- Do my protocols address potential bias?
- Is my communication being customized to the community?
Answering YES to these questions means you are building a system that organically supports and creates person-centric clinical trials.
Acclinate’s NOWINCLUDED™ engagement platform and e-DICT™ predictive analytics are the perfect complement to Lightship’s tailored approach to clinical trial execution that creates more access, choice, and equity. As such, both organizations are actively teaming up on ongoing projects in the United States to generate meaningful engagement within diverse groups of highly representative patients.
Conclusion
Clinical trials need more diversity. It can’t be said enough. However, as Benjamin Franklin once wrote, “Well done is better than well said.” In order to facilitate a greater level of action, Lightship, Acclinate, DiME, and clinical research organizations around the world have put together a comprehensive collection of resources and digital tools that make transitioning to a more inclusive and person-centric approach as simple and straightforward as possible. The time to take action is now, and those who take action right away will be better prepared to execute their clinical trials well into the future. They will build trust within their communities and better understand each patient, all while producing more authentic and useful data sets.
Join us in shining a light on clinical research disparity. Access resources that will confidently guide your trial design toward a more inclusive, diverse, and equitable approach.
References
1 Algeria, M., Sudd, S., & Steinberg, B., et al. Reporting of Participant Race, Sex, and Socioeconomic Status in Randomized Clinical Trials in General Medical Journals, 2015 vs 2019. Jamal Network Open. Published 2021 May.
2 National Institute of Diabetes and Digestive Kidney Diseases. Race, Ethnicity, & Kidney Disease. U.S. Department of Health and Human Services. Last reviewed 2014 March.
3 Lucas, J., & Villarroel, M. Telemedicine Use Among Adults: United States, 2021. Centers for Disease Control and Prevention. Published 2022 Oct.